Multiple Choice
What is the energy (E, in J) of the photons emitted by an Ar laser with a wavelength of 488 nm?
A) 2.46 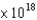 J
J
B) 9.69 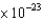 J
J
C) 1.36 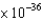 J
J
D) 4.07 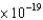 J
J
E) 2.46 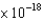 J
J
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q45: `If cesium, which has a work function
Q46: Which of the following atoms has no
Q47: What is the kinetic energy of the
Q48: Which of the transitions in the hydrogen
Q49: What is the minimum energy that a
Q51: A certain shell is known to have
Q52: Across a row in the periodic table,
Q53: In quantum mechanics, an atomic orbital _<br>A)provides
Q54: Which of the following is the ground-state
Q55: Which arrangement is in the correct order