Multiple Choice
Across a row in the periodic table, the ionization energy ________
A) increases because the n quantum number changes.
B) increases because the 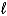 quantum number changes.
quantum number changes.
C) decreases because the effective nuclear charge decreases.
D) increases because the effective nuclear charge increases.
E) changes little because the outer electrons are all in the same shell.
Correct Answer:

Verified
Correct Answer:
Verified
Q47: What is the kinetic energy of the
Q48: Which of the transitions in the hydrogen
Q49: What is the minimum energy that a
Q50: What is the energy (E, in J)
Q51: A certain shell is known to have
Q53: In quantum mechanics, an atomic orbital _<br>A)provides
Q54: Which of the following is the ground-state
Q55: Which arrangement is in the correct order
Q56: Which one of the following diagrams represents
Q57: According to de Broglie, if the circumference