Multiple Choice
Indicate which metal requires the shortest wavelength photons to eject photoelectrons based on the following graph. 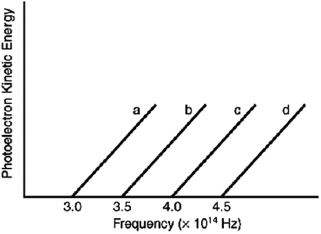
A) a
B) b
C) c
D) d
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q68: What is the ground state electron configuration
Q69: Which of the following metals would be
Q70: What is the photon energy of the
Q71: The Fe<sup>3</sup><font face="symbol"><sup></sup></font> transition metal ion has
Q72: Which of the following is not a
Q74: What are the principal and angular momentum
Q75: Which monatomic ion most likely exists?<br>A)Na<sup>2</sup><font face="symbol"><sup></sup></font><br>B)Ca<font
Q76: Early photoelectric light detectors were based on
Q77: Radiation with a wavelength of _ nm
Q78: Which of the following photons has the