Multiple Choice
What is the photon energy of the yellow-orange light ( 589 nm) produced by sodium vapor streetlights?
A) 3.37 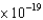 J
J
B) 6.63 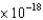 J
J
C) 2.99 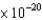 J
J
D) 1.45 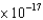 J
J
E) 7.45 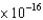 J
J
Correct Answer:

Verified
Correct Answer:
Verified
Q65: Which of the transitions in the following
Q66: Which combination of quantum numbers is possible
Q67: What is the wavelength (<font face="symbol"></font>, in
Q68: What is the ground state electron configuration
Q69: Which of the following metals would be
Q71: The Fe<sup>3</sup><font face="symbol"><sup></sup></font> transition metal ion has
Q72: Which of the following is not a
Q73: Indicate which metal requires the shortest wavelength
Q74: What are the principal and angular momentum
Q75: Which monatomic ion most likely exists?<br>A)Na<sup>2</sup><font face="symbol"><sup></sup></font><br>B)Ca<font