Multiple Choice
Which combination of quantum numbers is possible for an atom with five orbitals in one subshell?
A) n  1,
1, 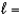 0
0
B) n  2,
2, 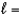 4
4
C) n  3,
3, 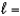 2
2
D) n  4,
4, 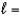 4
4
E) n  5,
5, 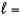 0
0
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q61: Arrange the following ions in order of
Q62: Determine the wavelength of the line in
Q63: If the principal quantum number is seven
Q64: What is the speed of an argon
Q65: Which of the transitions in the following
Q67: What is the wavelength (<font face="symbol"></font>, in
Q68: What is the ground state electron configuration
Q69: Which of the following metals would be
Q70: What is the photon energy of the
Q71: The Fe<sup>3</sup><font face="symbol"><sup></sup></font> transition metal ion has