Multiple Choice
Which of the following is a possible set of quantum numbers for a 3d orbital?
A) n  3,
3, 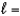 1, me
1, me
 1
1
B) n  3,
3, 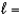 0, me
0, me  0
0
C) n  3,
3, 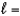 2, me
2, me  3
3
D) n  3,
3, 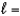 2, me
2, me  0
0
E) n  3,
3, 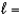 1, me
1, me  2
2
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q10: What is the orbital designation for an
Q11: How much energy is required to ionize
Q12: Which orbital has the highest energy in
Q13: A radio station's operating frequency has a
Q14: Which statement about the quantum numbers that
Q16: Which transition in a hydrogen atom requires
Q17: Astronomers have detected hydrogen atoms in interstellar
Q18: Which orbital does not fill before a
Q19: How much energy is required to ionize
Q20: Write the electron configuration of Co<sup>2</sup><font face="symbol"><sup></sup></font>.