Multiple Choice
Which statement about the quantum numbers that identify an atomic orbital is not correct?
A) The angular momentum quantum number, 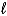 , identifies the shape of an orbital.
, identifies the shape of an orbital.
B) The value of the angular momentum quantum number can range from 0 to n, where n is the principal quantum number for the orbital.
C) Orbitals with the same value for the principal quantum number and the angular momentum quantum number are said to be in the same subshell.
D) Orbitals with the same values for the principal quantum number and the angular momentum quantum number have the same energy.
E) The value for the angular momentum quantum number also is designated by a letter: s 0, p 1, d 2, f 3, and so on.
Correct Answer:

Verified
Correct Answer:
Verified
Q9: If each of the following metals is
Q10: What is the orbital designation for an
Q11: How much energy is required to ionize
Q12: Which orbital has the highest energy in
Q13: A radio station's operating frequency has a
Q15: Which of the following is a possible
Q16: Which transition in a hydrogen atom requires
Q17: Astronomers have detected hydrogen atoms in interstellar
Q18: Which orbital does not fill before a
Q19: How much energy is required to ionize