Multiple Choice
Which of the following is a possible set of quantum numbers for a 4p orbital?
A) n  4,
4, 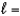 2, me
2, me
 1
1
B) n  4,
4, 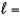 1, me
1, me  2
2
C) n  4,
4, 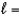 1, me
1, me  1
1
D) n  4,
4, 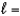 0, me
0, me  0
0
E) None could represent a 4p orbital.
Correct Answer:

Verified
Correct Answer:
Verified
Q75: Which monatomic ion most likely exists?<br>A)Na<sup>2</sup><font face="symbol"><sup></sup></font><br>B)Ca<font
Q76: Early photoelectric light detectors were based on
Q77: Radiation with a wavelength of _ nm
Q78: Which of the following photons has the
Q79: Two elements that have np<sup>3</sup> in their
Q81: De Broglie reasoned that for the electron
Q82: The work function of sodium is <font
Q83: Which of the following orbitals is the
Q84: Which of the following atoms or ions
Q85: What is the frequency (<font face="symbol"></font>, in