Multiple Choice
Which of the following orbitals is the lowest in energy (assuming they are all in the same shell) ?
A) 
B) 
C) 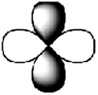
D) 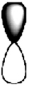
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q78: Which of the following photons has the
Q79: Two elements that have np<sup>3</sup> in their
Q80: Which of the following is a possible
Q81: De Broglie reasoned that for the electron
Q82: The work function of sodium is <font
Q84: Which of the following atoms or ions
Q85: What is the frequency (<font face="symbol"></font>, in
Q86: The <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3835/.jpg" alt="The 2
Q87: Place these types of radiation in order
Q88: Arrange the following elements in order of