Multiple Choice
Which listing has the orbitals in order of increasing energy in a multielectron atom?
A) 3s  3p
3p  3d
3d  4s
4s
B) 5s  4d
4d  5p
5p
C) 5s  3d
3d  5p
5p
D) 2px  2py
2py  2pz
2pz
E) 3s 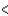 2p
2p 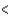 4s
4s
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q2: Which of the following is not a
Q3: How many electrons can be in the
Q4: Which of the transitions in the hydrogen
Q5: Consider the radial distribution shown below for
Q6: Which of the following elements would you
Q8: Which of the following sources produces the
Q9: If each of the following metals is
Q10: What is the orbital designation for an
Q11: How much energy is required to ionize
Q12: Which orbital has the highest energy in