Multiple Choice
If each of the following metals is exposed to light with a wavelength of 240 nm, which will emit photoelectrons with the greatest kinetic energy?
A) iron ( 7.2 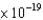 J)
J)
B) platinum ( 9.1 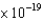 J)
J)
C) nickel ( 8.3 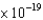 J)
J)
D) palladium ( 8.2 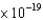 J)
J)
E) sodium ( 4.4 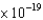 J)
J)
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q4: Which of the transitions in the hydrogen
Q5: Consider the radial distribution shown below for
Q6: Which of the following elements would you
Q7: Which listing has the orbitals in order
Q8: Which of the following sources produces the
Q10: What is the orbital designation for an
Q11: How much energy is required to ionize
Q12: Which orbital has the highest energy in
Q13: A radio station's operating frequency has a
Q14: Which statement about the quantum numbers that