Multiple Choice
Down a column in the periodic table, the ionization energy ________
A) increases because the n quantum number changes.
B) increases because the 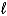 quantum number changes.
quantum number changes.
C) decreases because the outer electrons are further from the nucleus.
D) increases because the atomic number (nuclear charge) increases.
E) changes little because the outer electrons are all in the same outer shell.
Correct Answer:

Verified
Correct Answer:
Verified
Q139: Which of the following elements has the
Q140: Which arrangement is not in the correct
Q141: How many orbitals are possible for the
Q142: What wavelength of light is required to
Q143: Based on the following graph, which metal
Q145: What is the energy (E, in J)
Q146: Define effective nuclear charge. Use this parameter
Q147: A light detector based on the photoelectric
Q148: The mathematical description of an electron as
Q149: Which monatomic ion most likely does not