Multiple Choice
Which arrangement is not in the correct order of decreasing first ionization energy?
A) B 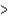 Al
Al 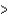 In
In
B) As 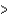 P
P 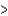 N
N
C) Cl 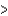 Si
Si 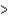 Na
Na
D) Li 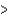 K
K 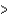 Cs
Cs
E) Br 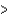 As
As 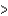 Ga
Ga
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q135: The emission spectra of Na and Na<font
Q136: Which of the following photons has the
Q137: Which subshell only has five orbitals?<br>A) <img
Q138: Identify which element, potassium or bromine, has
Q139: Which of the following elements has the
Q141: How many orbitals are possible for the
Q142: What wavelength of light is required to
Q143: Based on the following graph, which metal
Q144: Down a column in the periodic table,
Q145: What is the energy (E, in J)