Multiple Choice
Assuming that the distance between ions remains constant in all cases, which of the following ion pairs has the greatest electrostatic potential energy (i.e., largest in magnitude) ?
A) 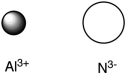
B) 
C) 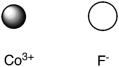
D) 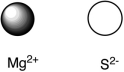
E) 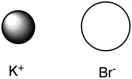
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q32: Which of the following objects will cool
Q33: In an experiment, 5.0 g of ice
Q34: Energy that an object has by virtue
Q35: What will be the final temperature of
Q36: Which of the following bar charts shows
Q38: A cooling curve for some substance is
Q39: The diagram below shows three ion pairs:
Q40: A cooling curve for some substance is
Q41: During a(n) _ process, energy is transferred
Q42: Write the equation that corresponds to the