Multiple Choice
Which of the following objects will cool the fastest from the same initial temperature, assuming you had equal masses of each?
A) an aluminum pan (cs 0.90 J/(g 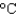 ) )
) )
B) a copper pot (cs 0.39 J/(g 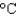 ) )
) )
C) an iron skillet (cs 0.45 J/(g 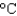 ) )
) )
D) a container of water (4.2 J/(g 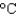 ) )
) )
E) a container of ethanol (2.5 J/(g  ) )
) )
Correct Answer:

Verified
Correct Answer:
Verified
Q27: Explain what is meant by the term
Q28: Isooctane is a good model compound for
Q29: Ethanol (CH<sub>3</sub>CH<sub>2</sub>OH) has been suggested as an
Q30: According to Coulomb's law, which ionic compound
Q31: Which statement A-D about a state function
Q33: In an experiment, 5.0 g of ice
Q34: Energy that an object has by virtue
Q35: What will be the final temperature of
Q36: Which of the following bar charts shows
Q37: Assuming that the distance between ions remains