Multiple Choice
Ammonium nitrate sometimes is used in cold packs because its enthalpy of solvation in water is 21 kJ/mol. What is the minimum amount of ammonium nitrate (80.05 g/mol) that is needed to cool a 1.0 L soft drink (d 1.0 g/mL, specific heat 4.5 J g1 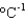 ) and its plastic bottle (heat capacity 50 J/K) from 30
) and its plastic bottle (heat capacity 50 J/K) from 30 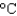 to 5
to 5 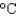 .
.
A) 540 g
B) 320 g
C) 110 g
D) 430 g
E) 250 g
Correct Answer:

Verified
Correct Answer:
Verified
Q52: According to Coulomb's law, which ionic compound
Q53: In an experiment, 30.0 g of metal
Q54: Suppose you eat a one-third pound hamburger
Q55: Fuel density is _<br>A)the cost of energy
Q56: Which statement regarding combustion of a sample
Q58: Given the following thermochemical equation detailing the
Q59: How much work does a gas do
Q60: The kinetic energy associated with the random
Q61: Which of the following is an endothermic
Q62: C<sub>8</sub>H<sub>18</sub> (114 g/mol, d <font face="symbol"></font> 0.69