Multiple Choice
Given the following thermochemical equation detailing the combustion of glucose C6H12O6(s) 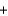 6O2(g)
6O2(g)  CO2(g)
CO2(g) 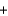 6H2O(g) Hrxn 2803 kJ/mol C6H12O6
6H2O(g) Hrxn 2803 kJ/mol C6H12O6
Determine the amount of glucose needed to release 30 kJ of energy.
A) 11.58 g
B) 1.93 g
C) 467 g
D) 1,930 g
E) 5.35 g
Correct Answer:

Verified
Correct Answer:
Verified
Q53: In an experiment, 30.0 g of metal
Q54: Suppose you eat a one-third pound hamburger
Q55: Fuel density is _<br>A)the cost of energy
Q56: Which statement regarding combustion of a sample
Q57: Ammonium nitrate sometimes is used in cold
Q59: How much work does a gas do
Q60: The kinetic energy associated with the random
Q61: Which of the following is an endothermic
Q62: C<sub>8</sub>H<sub>18</sub> (114 g/mol, d <font face="symbol"></font> 0.69
Q63: You heat a cup of coffee in