Multiple Choice
Given the standard enthalpies of formation for the following substances, determine the reaction enthalpy for the following reaction. 2N2H4(g) 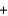 2NO2(g)
2NO2(g)  3N2(g)
3N2(g) 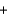 4H2O(g) Hrxn ? kJ
4H2O(g) Hrxn ? kJ
SubstanceH 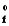 in kJ/mol
in kJ/mol
N2H4(g) 95.4
NO2(g) 33.1
H2O(g) 241.8
A) (1,220 kJ)
B) (1,220 kJ)
C) (967 kJ/mol)
D) (967 kJ/mol)
E) (257 kJ/mol)
Correct Answer:

Verified
Correct Answer:
Verified
Q3: Ethanol (CH<sub>3</sub>CH<sub>2</sub>OH) has been suggested as an
Q4: Which of the following hydrocarbons has the
Q5: Which statement A-D about energy units is
Q6: Sports trainers use cold packs containing ammonium
Q7: Which of the following fuels has the
Q9: What is the kinetic energy of a
Q10: Which of the changes A-D will always
Q11: If a chemical reaction causes the temperature
Q12: An expanding gas does 175 kJ of
Q13: When solid sodium hydroxide (NaOH) pellets are