Short Answer
Sports trainers use cold packs containing ammonium nitrate for injured athletes. Calculate the change in temperature when 47 g of ammonium nitrate (NH4NO3, 80.1 g/mol) dissolves in 100 g water. Assume the specific heat of the solution is 4.5 J/(g C).
NH4NO3(s)  NH4(aq)
NH4(aq) 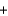 NO3(aq) H 21.1 kJ/mol
NO3(aq) H 21.1 kJ/mol
Correct Answer:

Verified
F...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q1: The following diagrams illustrate the flow of
Q2: Thermochemistry is the study of how _
Q3: Ethanol (CH<sub>3</sub>CH<sub>2</sub>OH) has been suggested as an
Q4: Which of the following hydrocarbons has the
Q5: Which statement A-D about energy units is
Q7: Which of the following fuels has the
Q8: Given the standard enthalpies of formation for
Q9: What is the kinetic energy of a
Q10: Which of the changes A-D will always
Q11: If a chemical reaction causes the temperature