Short Answer
Given the following thermochemical equation detailing the combustion of methane
CH4(g) 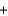 2O2(g)
2O2(g)  CO2(g)
CO2(g) 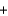 2H2O(g) Hrxn 802 kJ/mol CH4
2H2O(g) Hrxn 802 kJ/mol CH4
determine the amount of energy released when 25 g of methane undergoes combustion.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q75: The energy content of a Big Mac
Q76: During an exothermic process, _ for the
Q77: Work is done when a force moves
Q78: Which statement below regarding the various heat
Q79: What is the change in internal energy
Q81: Which statement A-D about the first law
Q82: Which statement A-D about the relationship between
Q83: In an experiment, 74.3 g of metallic
Q84: A diver jumps off a diving board.
Q85: During the expansion of steam inside the