Multiple Choice
The energy content of a Big Mac is 540 Cal. How much water can be heated from 20 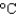 to 90
to 90 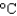 by this amount of energy? (cP(water) 1.00 g/mL, cs(water) 4.18 J/(g
by this amount of energy? (cP(water) 1.00 g/mL, cs(water) 4.18 J/(g 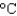 ) , 1 Cal 1,000 cal 4.184 kJ)
) , 1 Cal 1,000 cal 4.184 kJ)
A) 2.2 mL
B) 6.0 L
C) 7.7 mL
D) 7.7 L
E) 6.0 mL
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q70: In an experiment, 7.5 g of liquid
Q71: Describe the difference between potential energy and
Q72: Motor fuels are mixtures of hydrocarbons, but
Q73: Which statement regarding combustion of a sample
Q74: The basal metabolic rate (BMR) is the
Q76: During an exothermic process, _ for the
Q77: Work is done when a force moves
Q78: Which statement below regarding the various heat
Q79: What is the change in internal energy
Q80: Given the following thermochemical equation detailing the