Short Answer
Given the standard enthalpies of formation for the following substances, determine the reaction enthalpy for the following reaction.
2N2H4(g) 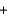 2NO2(g)
2NO2(g)  3N2(g)
3N2(g) 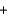 4H2O(g) Hrxn ? kJ
4H2O(g) Hrxn ? kJ
SubstanceH  in kJ/mol
in kJ/mol
N2H4(g)95.4
NO2(g)33.1
H2O(g)241.8
Correct Answer:

Verified
F...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q97: The following diagrams illustrate the flow of
Q98: When 2.50 g of sucrose (molar mass
Q99: In a Reuters news report dated February
Q100: The first law of thermodynamics implies that
Q101: When ammonium nitrate (NH<sub>4</sub>NO<sub>3</sub>)(s) is used in
Q103: Which arrow in the following diagrams represents
Q104: Use the following information to determine the
Q105: Internal energy is defined as _<br>A)the total
Q106: Predict the temperature change produced by burning
Q107: You hold a 50 g sphere of