Multiple Choice
You hold a 50 g sphere of copper in one hand and a 25 g sphere of aluminum in the other hand. If both absorb energy at the same rate, which will come to your body temperature first and why? The specific heat capacities are 0.4 J/(g 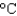 ) for copper and 0.9 J/(g
) for copper and 0.9 J/(g 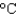 ) for aluminum.
) for aluminum.
A) copper, because the specific heat is smaller
B) aluminum, because the specific heat is larger
C) aluminum, because the mass is smaller
D) copper, because the heat capacity is smaller
E) Both reach body temperature at the same time because they absorb energy at the same rate.
Correct Answer:

Verified
Correct Answer:
Verified
Q102: Given the standard enthalpies of formation for
Q103: Which arrow in the following diagrams represents
Q104: Use the following information to determine the
Q105: Internal energy is defined as _<br>A)the total
Q106: Predict the temperature change produced by burning
Q108: Which arrow in the following diagrams represents
Q109: Assuming that the distances between the two
Q110: In terms of the enthalpy of formation,
Q111: What is the heat capacity (C<sub>p</sub>) of
Q112: Heat is best defined as _<br>A)a substance