Multiple Choice
How many moles of magnesium nitrate are present in 75.0 mL of a 0.33 M solution?
A) 2.5 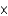
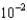 moles
moles
B) 25 moles
C) 4.4 moles
D) 2.3 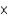
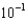 moles
moles
E) 4.4 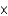
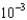 moles
moles
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q65: In a titration, the solution of known
Q66: Some microorganisms living under anaerobic conditions extract
Q67: Which of the following compounds are soluble
Q68: Human blood contains about 0.18 g potassium
Q69: Sodium fluoride (NaF) is added to drinking
Q71: The drinking water standard of the
Q72: Which one of the following water solubility
Q73: How many grams of solid magnesium chloride,
Q74: What volume of 3.0 M NaOH contains
Q75: What are the units of molar concentration,