Multiple Choice
Sodium fluoride (NaF) is added to drinking water in some municipalities to protect teeth against cavities. The idea is to convert hydroxyapatite, Ca10(PO4) 6(OH) 2, into more stable fluorapatite, Ca10(PO4) 6F2. The molar mass of hydroxyapatite is 502 g/mol. What is the molarity of a 10.0 mg/L sodium fluoride solution?
A) 2.38 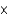
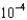 M
M
B) 1.99 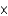
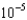 M
M
C) 2.63 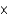
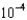 M
M
D) 1.19 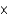
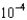 M
M
E) 0.263 M
Correct Answer:

Verified
Correct Answer:
Verified
Q64: Which one of the following is the
Q65: In a titration, the solution of known
Q66: Some microorganisms living under anaerobic conditions extract
Q67: Which of the following compounds are soluble
Q68: Human blood contains about 0.18 g potassium
Q70: How many moles of magnesium nitrate are
Q71: The drinking water standard of the
Q72: Which one of the following water solubility
Q73: How many grams of solid magnesium chloride,
Q74: What volume of 3.0 M NaOH contains