Multiple Choice
Which one of the following reaction equations is the net ionic equation for the reaction of hydrochloric acid with lithium hydroxide? All species are in aqueous solution.
A) HCl 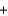 LiOH
LiOH  LiCl
LiCl 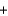 H2O
H2O
B) H+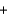 Cl-
Cl-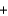 Li+
Li+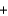 OH-
OH- Li+
Li+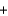 Cl-
Cl-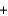 H2O
H2O
C) H+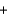 Cl-
Cl-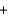 Li+ OH-
Li+ OH- LiCl
LiCl 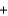 H2O
H2O
D) Li+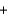 Cl-
Cl- LiCl
LiCl
E) H+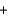 OH-
OH- H2O
H2O
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q71: The drinking water standard of the
Q72: Which one of the following water solubility
Q73: How many grams of solid magnesium chloride,
Q74: What volume of 3.0 M NaOH contains
Q75: What are the units of molar concentration,
Q77: In going from the top to the
Q78: What are the spectator ions in the
Q79: Determine the molar concentration of an aqueous
Q80: Which of the following ionic compounds is
Q81: What mass of calcium hydroxide (molar mass