Multiple Choice
Determine the molar concentration of an aqueous solution of lead(II) nitrate solution that is 26 ppb (parts per billion) Pb(NO3) 2. Since the solution is very dilute, assume the density is that of water, 1.00 g/mL.
A) 7.85 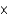
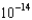 M
M
B) 2.60 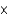
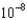 M
M
C) 4.26 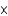
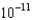 M
M
D) 7.85 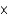
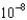 M
M
E) 7.85 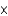
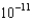 M
M
Correct Answer:

Verified
Correct Answer:
Verified
Q74: What volume of 3.0 M NaOH contains
Q75: What are the units of molar concentration,
Q76: Which one of the following reaction equations
Q77: In going from the top to the
Q78: What are the spectator ions in the
Q80: Which of the following ionic compounds is
Q81: What mass of calcium hydroxide (molar mass
Q82: Silver salts are used in black-and-white photography.
Q83: The half-reaction for the reduction of molecular
Q84: Magnesium metal burns brightly in the air