Multiple Choice
A reaction that takes place between dissolved barium nitrate and dissolved sodium sulfate results in formation of solid barium sulfate. Which equation describes this reaction?
A) Ba(NO3) 2(aq) 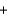 Na2SO4(aq)
Na2SO4(aq)  BaSO4(s)
BaSO4(s) 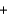 2NaNO3(aq)
2NaNO3(aq)
B) BaNO3(aq) 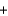 NaSO4(aq)
NaSO4(aq)  BaSO4(s)
BaSO4(s) 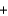 NaNO3(aq)
NaNO3(aq)
C) 2Ba(NO3) (aq) 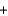 Na2SO4(aq)
Na2SO4(aq)  Ba2SO4(s)
Ba2SO4(s) 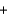 2NaNO3(aq)
2NaNO3(aq)
D) Ba(NO3) 2(aq) 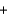 2NaSO4(aq)
2NaSO4(aq)  Ba(SO4) 2(s)
Ba(SO4) 2(s) 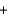 2NaNO3(aq)
2NaNO3(aq)
E) Ba(NO3) 2(aq) 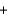 Na2SO4(aq)
Na2SO4(aq)  BaSO4(aq)
BaSO4(aq) 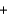 2NaNO3(s)
2NaNO3(s)
Correct Answer:

Verified
Correct Answer:
Verified
Q33: Which one of the following is not
Q34: What is the oxidation number of S
Q35: Which picture best represents an atomic-level view
Q36: According to the label on a bottle
Q37: Ion-exchange resins can remove Mg<sup>2+</sup> and Ca<sup>2+</sup>0
Q39: Concentrated sulfuric acid contains 4 g of
Q40: The oxidation number of carbon in carbon
Q41: Most chloride salts are soluble. Identify an
Q42: In the following reaction, which element or
Q43: In which one of the following compounds