Multiple Choice
In the following reaction, which element or ion is reduced? Cl2(g) 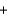 NaBr(aq)
NaBr(aq)  NaCl(aq)
NaCl(aq) 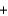 Br2(g)
Br2(g)
A) Br2
B) Na+
C) Cl2
D) Br -
E) C-
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q37: Ion-exchange resins can remove Mg<sup>2+</sup> and Ca<sup>2+</sup>0
Q38: A reaction that takes place between dissolved
Q39: Concentrated sulfuric acid contains 4 g of
Q40: The oxidation number of carbon in carbon
Q41: Most chloride salts are soluble. Identify an
Q43: In which one of the following compounds
Q44: In the following reaction, which element is
Q45: A chemistry student attempted to make a
Q46: Identify the base in the following acid-base
Q47: If 100 mL of 3.0 M solution