Multiple Choice
The half-reaction for the reduction of molecular oxygen to water is ________
A) O2(g)  H2O(l ) .
H2O(l ) .
B) O2(g) 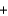 H2(g)
H2(g)  H2O(l ) .
H2O(l ) .
C) O2(g) 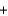 H2(g)
H2(g) 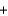 2e-
2e- H2O(l ) .
H2O(l ) .
D) O2(g) 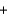 2 H+(g)
2 H+(g) 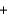 2e-
2e-  H2O(l ) .
H2O(l ) .
E) O2(g) 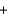 2 H+(g)
2 H+(g)  H2O(l )
H2O(l ) 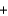 2e-.
2e-.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q78: What are the spectator ions in the
Q79: Determine the molar concentration of an aqueous
Q80: Which of the following ionic compounds is
Q81: What mass of calcium hydroxide (molar mass
Q82: Silver salts are used in black-and-white photography.
Q84: Magnesium metal burns brightly in the air
Q85: Identify the solution below that will conduct
Q86: Sodium fluoride is added to drinking water
Q87: What is the molar concentration of sodium
Q88: In which compound does chlorine have an