Multiple Choice
Which statement A-D regarding the terms mole and molar mass is not correct?
A) A mole is defined as the number of particles in exactly 12 g of carbon-12.
B) A mole of oxygen gas contains 6.022 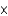 1023 molecules.
1023 molecules.
C) Two moles of oxygen atoms can be obtained by decomposing one mole of carbon dioxide.
D) To obtain the molar mass in grams (g/mol) from the atomic mass in atomic mass units(u) , multiply by 6.022 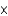 1023/mol and divide by 6.022
1023/mol and divide by 6.022 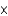 1023 u/g.
1023 u/g.
E) Statements A-D all are correct.
Correct Answer:

Verified
Correct Answer:
Verified
Q110: Ozone (O<sub>3</sub>) reacts with iodide (I<sup>-</sup>) and
Q111: One mole is defined as _<br>A)the number
Q112: Combustion analysis of an organic compound to
Q113: How many oxygen atoms are there in
Q114: Elemental analysis of the organic compound meta-xylene
Q116: Air bags in cars inflate when an
Q117: Allicin, a potent antibacterial compound, is formed
Q118: What is the difference in the chemistry
Q119: How many moles of ammonia are there
Q120: The combustion of fossil fuels is the