Multiple Choice
One mole is defined as ________
A) the number of particles equal to the number of atoms in exactly 12 g of carbon-12.
B) the number of particles equal to the number of atoms in exactly 16 g of oxygen-16.
C) exactly 6.02 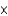 1022 particles.
1022 particles.
D) the number of particles equal to the number of atoms in exactly 1 g of hydrogen-1.
E) the number of particles equal to the number of atoms in exactly 1 kg of carbon in a vault at the National Bureau of Standards.
Correct Answer:

Verified
Correct Answer:
Verified
Q106: Which molecules shown below have the same
Q107: One form of asbestos called chrysotile is
Q108: Carbon monoxide is used in refining iron
Q109: Chlorhexidine is an antibacterial drug found in
Q110: Ozone (O<sub>3</sub>) reacts with iodide (I<sup>-</sup>) and
Q112: Combustion analysis of an organic compound to
Q113: How many oxygen atoms are there in
Q114: Elemental analysis of the organic compound meta-xylene
Q115: Which statement A-D regarding the terms mole
Q116: Air bags in cars inflate when an