Multiple Choice
How many chlorine atoms are there in 16.0 g of carbon tetrachloride (CCl4) ?
A) 6.91 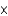 1025
1025
B) 1.04 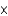 10-1
10-1
C) 6.26 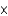 1022
1022
D) 2.51 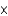 1023
1023
E) 4.23 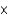 1017
1017
Correct Answer:

Verified
Correct Answer:
Verified
Q96: The acid-base reaction between phosphoric acid, H<sub>3</sub>PO<sub>4</sub>,
Q97: Beryllium metal reacts with chlorine gas to
Q98: U.S. Lime & Minerals is a company
Q99: Fool's Gold is the mineral pyrite (FeS<sub>2</sub>).
Q100: Which one of the following samples contains
Q102: A tiny speck (8.3 <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3835/.jpg" alt="A
Q103: Write a definition of the phrase "stoichiometry
Q104: Which substance listed below contains the most
Q105: What is the formula mass of (NH<sub>4</sub>)<sub>3</sub>PO<sub>4</sub>
Q106: Which molecules shown below have the same