Multiple Choice
Beryllium metal reacts with chlorine gas to produce beryllium chloride (BeCl2) . Given the following diagram of available beryllium atoms and chlorine molecules in a reaction flask, identify the excess reagent and how many atoms/molecules of reactant remain at the end of the reaction. 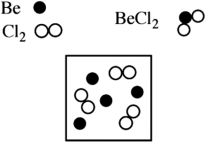
A) Excess reagent: chlorine; Molecules remaining: one
B) Excess reagent: beryllium; Atoms remaining: one
C) Excess reagent: chlorine; Molecules remaining: two
D) Excess reagent: beryllium; Atoms remaining: two
E) There is no excess reagent in this reaction.
Correct Answer:

Verified
Correct Answer:
Verified
Q92: Calcium hydride (CaH<sub>2</sub>) is so reactive with
Q93: A mass of 11.60 g of phosphoric
Q94: Sulfur dioxide from coal-fired power plants combines
Q95: Water can be separated into its elements
Q96: The acid-base reaction between phosphoric acid, H<sub>3</sub>PO<sub>4</sub>,
Q98: U.S. Lime & Minerals is a company
Q99: Fool's Gold is the mineral pyrite (FeS<sub>2</sub>).
Q100: Which one of the following samples contains
Q101: How many chlorine atoms are there in
Q102: A tiny speck (8.3 <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3835/.jpg" alt="A