Multiple Choice
Nitrogen monoxide undergoes combustion to produce nitrogen dioxide. Which relationship regarding the quantities of reactants and products associated with this reaction is not correct? 2NO 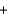 O2
O2  2NO2
2NO2
A) 2x g 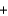 x g
x g  2x g
2x g
B) 2 molecules 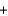 +1 molecule
+1 molecule  2 molecules
2 molecules
C) 60.0 g 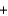 32.0 g
32.0 g  92.0 g
92.0 g
D) (2y) (30.0 g) 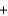 y (32.0 g)
y (32.0 g)  (2y) (46.0 g)
(2y) (46.0 g)
E) 15.0 g 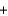 8.0 g
8.0 g  23.0 g
23.0 g
Correct Answer:

Verified
Correct Answer:
Verified
Q17: Like many metals, manganese reacts with halogens
Q18: Ammonia undergoes combustion to produce nitrogen monoxide
Q19: Which statements regarding combustion analysis to determine
Q20: How many total atoms are there in
Q21: Which of the following cartoons depict a
Q23: Without doing any calculations, identify which of
Q24: Balance the following chemical equation:<br>C<sub>2</sub>H<sub>3</sub>OCl <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3835/.jpg"
Q25: At Sal's Sandwich Shop, a regular club
Q26: When are an empirical formula and a
Q27: A compound known to contain platinum, nitrogen,