Short Answer
Like many metals, manganese reacts with halogens to give metal halides. How many grams of MnF3 can be produced using excess fluorine and 5.49 g of Mn? (molar masses: Mn = 54.9 g/mol,
F  19.0 g/mol)
19.0 g/mol)
2Mn(s) 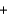 3F2(g)
3F2(g)  2MnF3(s)
2MnF3(s)
Correct Answer:

Verified
Correct Answer:
Verified
Q12: In one analysis, 3.01 <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3835/.jpg" alt="In
Q13: Acetaminophen is a common medication used to
Q14: Azobenzene is a highly colored organic compound.
Q15: For movie night, Annabelle popped two bags
Q16: Balance the following combustion reaction and report
Q18: Ammonia undergoes combustion to produce nitrogen monoxide
Q19: Which statements regarding combustion analysis to determine
Q20: How many total atoms are there in
Q21: Which of the following cartoons depict a
Q22: Nitrogen monoxide undergoes combustion to produce nitrogen