Multiple Choice
Which statement about the following chemical reaction is not correct? 3H2 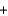
N2

2NH3
A) For every mole of nitrogen consumed, two moles of ammonia are produced.
B) For every two moles of nitrogen consumed, three moles of ammonia are produced.
C) For every three moles of hydrogen consumed, two moles of ammonia are produced.
D) For every two moles of ammonia produced, three moles of hydrogen are consumed.
E) Three moles of hydrogen will react with one mole of nitrogen to produce ammonia.
Correct Answer:

Verified
Correct Answer:
Verified
Q27: A compound known to contain platinum, nitrogen,
Q28: The average car emits 8.0 kg
Q29: Phosphorus pentoxide is a common drying agent
Q30: List three reasons why the actual yield
Q31: In one analysis, 1.34 <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3835/.jpg" alt="In
Q33: Dialuminum hexachloride, Al<sub>2</sub>Cl<sub>6</sub> (266.66 g/mol), is an
Q34: Fe<sub>2</sub>O<sub>3</sub>(s) and powdered aluminum can react with
Q35: Even though lead is toxic, lead compounds
Q36: A burner in a gas grill mixes
Q37: How many hydrogen atoms are there in