Multiple Choice
Dialuminum hexachloride, Al2Cl6 (266.66 g/mol) , is an inexpensive compound that is used in many industrial processes. It is made by treating scrap aluminum with chlorine gas. If a reaction is run with 270 g of aluminum and 710 grams of chlorine, the limiting reactant is ________, and the theoretical yield is ________ of Al2Cl6. 2Al(s) 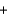 3Cl2(g)
3Cl2(g)  Al2Cl6(s)
Al2Cl6(s)
A) Al; 890 g
B) Cl2; 890 g
C) Al; 1,300 g
D) Cl2; 1,300 g
E) Cl2; 2,700 g
Correct Answer:

Verified
Correct Answer:
Verified
Q28: The average car emits 8.0 kg
Q29: Phosphorus pentoxide is a common drying agent
Q30: List three reasons why the actual yield
Q31: In one analysis, 1.34 <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3835/.jpg" alt="In
Q32: Which statement about the following chemical reaction
Q34: Fe<sub>2</sub>O<sub>3</sub>(s) and powdered aluminum can react with
Q35: Even though lead is toxic, lead compounds
Q36: A burner in a gas grill mixes
Q37: How many hydrogen atoms are there in
Q38: Diesel fuel for automobiles and trucks is