Short Answer
Carbon monoxide is used in refining iron ore to produce the metal. Assuming the reaction yield is 100%, how many grams of iron would be produced if 5.00 mol of carbon monoxide were exposed to 800 g iron(III) oxide?
Fe2O3(s) 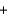 3CO(g)
3CO(g)  2Fe(s)
2Fe(s) 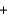 3CO2(g)
3CO2(g)
Correct Answer:

Verified
Correct Answer:
Verified
Q103: Write a definition of the phrase "stoichiometry
Q104: Which substance listed below contains the most
Q105: What is the formula mass of (NH<sub>4</sub>)<sub>3</sub>PO<sub>4</sub>
Q106: Which molecules shown below have the same
Q107: One form of asbestos called chrysotile is
Q109: Chlorhexidine is an antibacterial drug found in
Q110: Ozone (O<sub>3</sub>) reacts with iodide (I<sup>-</sup>) and
Q111: One mole is defined as _<br>A)the number
Q112: Combustion analysis of an organic compound to
Q113: How many oxygen atoms are there in