Multiple Choice
The molecular formula C2H4O can be converted into three-line bond (Kekulé) structures that are consistent with valence rules. Which one of the following Kekulé structures is not consistent with valence rules?
A) 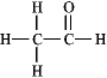
B) 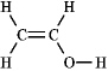
C) 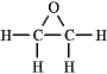
D) 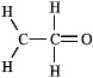
Correct Answer:

Verified
Correct Answer:
Verified
Q9: Instructions: Use the convention d-/d+ and the
Q10: Consider the formation of an sp<sup>2</sup> hybrid
Q11: Use the curved arrow method to
Q12: Instructions: Refer to the following equation to
Q13: Instructions: Determine the hybridization for the indicated
Q15: Write an equation for the reaction of
Q16: The following shows an intermediate used in
Q17: Specify the hybridization of each carbon atom
Q18: Instructions: Refer to the following equation to
Q19: Covalent bonding<br>A) involves a transfer of electrons