Essay
Specify the hybridization of each carbon atom of limonene, a natural product present in citrus fruits, and thujone, which is derived from wormwood, a traditional component of the notorious liquor, Absinthe.
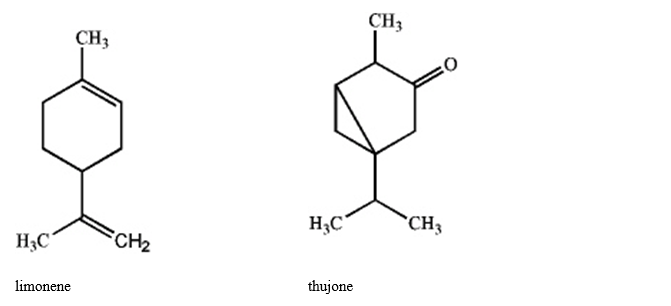
Correct Answer:

Verified
Correct Answer:
Verified
Q12: Instructions: Refer to the following equation to
Q13: Instructions: Determine the hybridization for the indicated
Q14: The molecular formula C<sub>2</sub>H<sub>4</sub>O can be converted
Q15: Write an equation for the reaction of
Q16: The following shows an intermediate used in
Q18: Instructions: Refer to the following equation to
Q19: Covalent bonding<br>A) involves a transfer of electrons
Q20: Which of the following statements is not
Q21: Instructions: Refer to the following equation to
Q22: The structure of urea is shown below.