Multiple Choice
In the following reaction in aqueous solution, the acid reactant is __________ and its conjugate base product is __________. CH3COOH + NH3 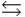 CH3COO- + NH4+
CH3COO- + NH4+
A) CH3COOH; CH3COO-
B) CH3COOH; NH4+
C) NH3; CH3COO-
D) NH3; NH4+
E) CH3COOH; H3O+
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q3: Which of the following can be mixed
Q20: The degree of ionization of a strong
Q44: What is indicated by the shape of
Q45: Phosphoric acid is a triprotic acid, ionizing
Q46: The acid ionization equilibrium constant, K<sub>a</sub>, describes
Q48: The following titration curve is most likely
Q50: Three acids found in foods are lactic
Q52: A solution with pH of 9.50 has
Q106: What is the pH of a 0.20
Q144: Which one of the following statements is