Multiple Choice
The acid ionization equilibrium constant, Ka, describes the reaction (where HA is a generic weak acid) :
A) HA + OH- 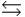 H2O + A-
H2O + A-
B) HA + H2O 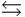 H3O+ + A-
H3O+ + A-
C) HA + H3O+ 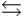 H2A+ + H2O
H2A+ + H2O
D) HA + H2A+ 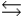 H2A+ + HA
H2A+ + HA
E) H3O+ + A- 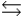 HA + H2O
HA + H2O
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q3: Which of the following can be mixed
Q20: The degree of ionization of a strong
Q41: Phosphoric acid is a triprotic acid, ionizing
Q44: What is indicated by the shape of
Q45: Phosphoric acid is a triprotic acid, ionizing
Q47: In the following reaction in aqueous solution,
Q48: The following titration curve is most likely
Q50: Three acids found in foods are lactic
Q106: What is the pH of a 0.20
Q144: Which one of the following statements is