Multiple Choice
Magnesium sulfate can be obtained at a drugstore as Epsom salts. The monohydrate is found as the mineral kieserite. What would be the pH of a 500 mL aqueous solution containing 1.62 g kieserite? The 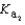 value for sulfuric acid is 1.2 10-2.
value for sulfuric acid is 1.2 10-2.
A) 7.00
B) 6.85
C) 7.15
D) 3.55
E) 12.22
Correct Answer:

Verified
Correct Answer:
Verified
Q7: To simulate the pH of blood, which
Q9: Which one, A-D, is not related to
Q16: Which combination of solutions is the best
Q39: A solution of hydrochloric acid (HCl, 25.00
Q45: If 500 mL of a solution containing
Q72: Research with biochemical systems commonly requires buffers
Q83: Which of the following groups, A-D, consist
Q102: When [H<sup>+</sup>] = 4.0 *10<sup>-</sup><sup>9</sup> M in
Q127: Purveyors of salts from the Dead Sea
Q129: Which one of the following salts forms