Multiple Choice
Phosphoric acid is a triprotic acid, ionizing in the following sequential steps: H3PO4 + H2O 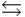 H2PO4- + H3O+
H2PO4- + H3O+ 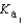 H2PO4- + H2O
H2PO4- + H2O 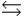 HPO42- + H3O+
HPO42- + H3O+ 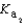 HPO42- + H2O
HPO42- + H2O 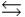 PO43- + H3O+
PO43- + H3O+ 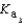 Which equilibrium is most important in determining the pH of a solution of sodium phosphate?
Which equilibrium is most important in determining the pH of a solution of sodium phosphate?
A) HPO42- + H2O 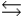 PO43- + H3O+
PO43- + H3O+
B) H3PO4 + H2O 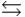 H2PO4- + H3O+
H2PO4- + H3O+
C) PO43- + H2O 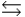 HPO42- + OH-
HPO42- + OH-
D) H2PO4- + H2O 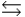 H3PO4 + OH-
H3PO4 + OH-
E) 2H2O 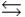 H3O+ + OH-
H3O+ + OH-
Correct Answer:

Verified
Correct Answer:
Verified
Q3: Which of the following can be mixed
Q10: What is the solubility of barium sulfate
Q27: In each of the following, the stronger
Q37: Which sketch best represents the qualitative molecular
Q39: Which sketch best represents the qualitative molecular
Q44: What is indicated by the shape of
Q45: Phosphoric acid is a triprotic acid, ionizing
Q46: The acid ionization equilibrium constant, K<sub>a</sub>, describes
Q115: Four solutions have pH values of 3,
Q144: Which one of the following statements is