Multiple Choice
Indicate which one of the following reactions result in a positive Ssys.
A) AgNO3(aq) + NaCl(aq) AgCl(s) + NaNO3(aq)
B) HCl(g) + H2O( 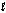 ) HCl(aq)
) HCl(aq)
C) H2(g) + I2(g) 2 Hl(g)
D) C2H2O2(g) 2 CO(g) + H2(g)
E) H2O(g) H2O(l)
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q9: The entropy change of the surroundings,
Q19: Which, if any, of A through D
Q21: Hydrochloric acid (HCl) reacts with sodium hydroxide
Q27: In a spontaneous process, the entropy of
Q28: A reaction is at equilibrium at a
Q41: The gas above the liquid in
Q57: The heat of fusion for water is
Q64: Dinitrogen tetroxide (N<sub>2</sub>O<sub>4</sub>) decomposes to nitrogen
Q87: The dissolution of ammonium nitrate in water
Q122: Which of the following must be true