Multiple Choice
A reaction is at equilibrium at a given temperature and constant pressure when __________
A) "Srxn" = 0.
B) "S 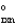 " = 0.
" = 0.
C) "Grxn = 0.
D) " G 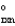 " = 0.
" = 0.
E) "Hrxn" = 0.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q9: The entropy change of the surroundings,
Q24: Indicate which one of the following reactions
Q27: In a spontaneous process, the entropy of
Q31: If a reaction A <font face="symbol"></font> B
Q32: Which of the following processes will lead
Q33: In a spontaneous process, which of the
Q41: The gas above the liquid in
Q53: At 0 K, the entropy of a
Q57: The heat of fusion for water is
Q64: Dinitrogen tetroxide (N<sub>2</sub>O<sub>4</sub>) decomposes to nitrogen