Multiple Choice
The symbol G  (CH4, g) refers to which of the following reactions?
(CH4, g) refers to which of the following reactions?
A) CH4(l) CH4(g)
B) CH4(g) CH4( 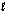 )
)
C) CH4(g) C(g) + 4H(g)
D) C(g) + 4H(g) CH4(g)
E) C(s, graphite) + 2H2(g) CH4(g)
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q15: At what temperature does the Fe(s)
Q35: "<font face="symbol"></font>S<sub>sys</sub>" can be directly related to
Q36: Determine the normal melting point of benzoic
Q37: The enthalpy of fusion for benzene is
Q44: Heat transfer from the system to the
Q57: The heat of fusion for water is
Q57: Name and state the first three laws
Q62: According to the second law of thermodynamics,
Q95: A reaction with a low enthalpy
Q146: The entropy of a NaCl crystal is