Short Answer
Determine the normal melting point of benzoic acid in degrees centigrade given the following data.
H 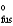 = 18.02 kJ/mol
= 18.02 kJ/mol
S 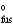 = 45.56 J/mol · K
= 45.56 J/mol · K
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q31: If a reaction A <font face="symbol"></font> B
Q32: Which of the following processes will lead
Q33: In a spontaneous process, which of the
Q35: "<font face="symbol"></font>S<sub>sys</sub>" can be directly related to
Q37: The enthalpy of fusion for benzene is
Q40: The symbol <font face="symbol"></font>G <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3833/.jpg" alt="The
Q57: The heat of fusion for water is
Q79: The entropy of vaporization of water is
Q95: A reaction with a low enthalpy
Q146: The entropy of a NaCl crystal is