Multiple Choice
The relative energies (strengths) of the intermolecular forces present in each of four different pure gases are shown in the figure below. Which gas will show the smallest deviation from ideal gas behavior? 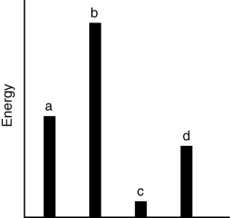
A) a
B) b
C) c
D) d
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q40: Arrange the first four halogens in order
Q46: Given the van der Waals a constant
Q48: Which alcohol should be most soluble in
Q76: Henry's law constant for oxygen dissolving in
Q77: Which statement about the phase diagram below
Q79: The relative energies (strengths) of the intermolecular
Q81: Point d in the phase diagram below
Q82: Which of the following diagrams best shows
Q110: Given the van der Waals a constant
Q141: Which of the following pairs of compounds